Prescription digital therapeutics are reshaping how we treat medical conditions through software. Unlike wellness apps that track steps or offer meditation guidance, prescription digital therapeutics are clinically validated interventions, approved or cleared by regulatory bodies like the FDA, and prescribed by a licensed healthcare provider. They are developed to treat, manage, or prevent specific diseases, often targeting chronic or behavioral health conditions, using evidence-based protocols delivered through mobile apps, wearables, or digital platforms.
What makes prescription digital therapeutics different is their medical-grade intent. These solutions undergo rigorous testing for safety, efficacy, and regulatory compliance, often playing a complementary role alongside pharmaceuticals or behavioral therapies. In contrast, wellness apps focus on general lifestyle improvements and are not subjected to the same clinical or regulatory scrutiny.
As healthcare embraces digital transformation, PDTs represent a critical bridge between consumer-grade health tools and formal clinical interventions, access, adherence, and long-term outcomes.
The digital health landscape has matured rapidly over the past decade. What began as a wave of fitness trackers and wellness apps has evolved into a clinically oriented ecosystem, where software isn’t just assisting care—it’s becoming the care. Prescription digital therapeutics sit at the center of this shift, offering a regulated, evidence-backed path to managing medical conditions through digital means.
Payers are increasingly investing in solutions that demonstrate measurable outcomes. Unlike general wellness apps, PDTs are backed by clinical studies and real-world data, making them more viable for reimbursement under existing and emerging payer frameworks.
Pharmacy benefit managers (PBMs) and health systems are creating digital formularies—curated lists of approved digital therapeutics. This institutional recognition opens formal reimbursement pathways and solidifies PDTs as part of the broader healthcare toolkit.
Wellness products rarely move beyond user engagement metrics. PDTs, however, are validated through randomized controlled trials, behavioral outcome studies, and adherence metrics. This alignment with traditional medical expectations makes them more attractive to providers, insurers, and regulators.
For digital health companies, shifting from “apps that engage” to “apps that heal” presents a powerful differentiator. Building prescription digital therapeutics allows product teams to:
In other words, PDTs aren’t just apps—they’re interventions. And that distinction opens up entirely new business models rooted in clinical value and medical legitimacy.
As digital health companies look to scale sustainably and win payer trust, building prescription digital therapeutics is no longer optional—it’s a strategic imperative.
One of the defining features of prescription digital therapeutics is that they are regulated medical products, subject to oversight by the U.S. Food and Drug Administration (FDA) or equivalent authorities in other markets. Navigating this path is complex but essential for unlocking the credibility, reimbursement, and scalability that PDTs promise.
Prescription digital therapeutics are a specific type of Software as a Medical Device (SaMD)—software intended to diagnose, treat, or manage a medical condition without being part of a hardware medical device.
The FDA classifies and reviews SaMD products using a risk-based framework. PDTs typically fall into Class II (moderate risk), requiring a clear demonstration of safety, efficacy, and intended use.
Depending on novelty and clinical risk, a PDT may go through:
Understanding where your product fits early in development is key to choosing the right validation and submission strategy.
Here are three standout examples that have successfully navigated the regulatory process:
These examples highlight the diversity of use cases and the growing maturity of the regulatory landscape for PDTs.
Related read: Redefining Measurement-Based Care in Behavioral Health
While the opportunity around prescription digital therapeutics is enormous, building and launching a successful PDT comes with unique challenges. Many product teams underestimate the shift from “software that supports care” to “software that is care”—until they hit regulatory, clinical, or go-to-market roadblocks.
Below are the most common pain points teams encounter and how to think about solving them.
The challenge:
How to navigate it:
The challenge:
How to navigate it:
The challenge:
How to navigate it:
The challenge:
How to navigate it:
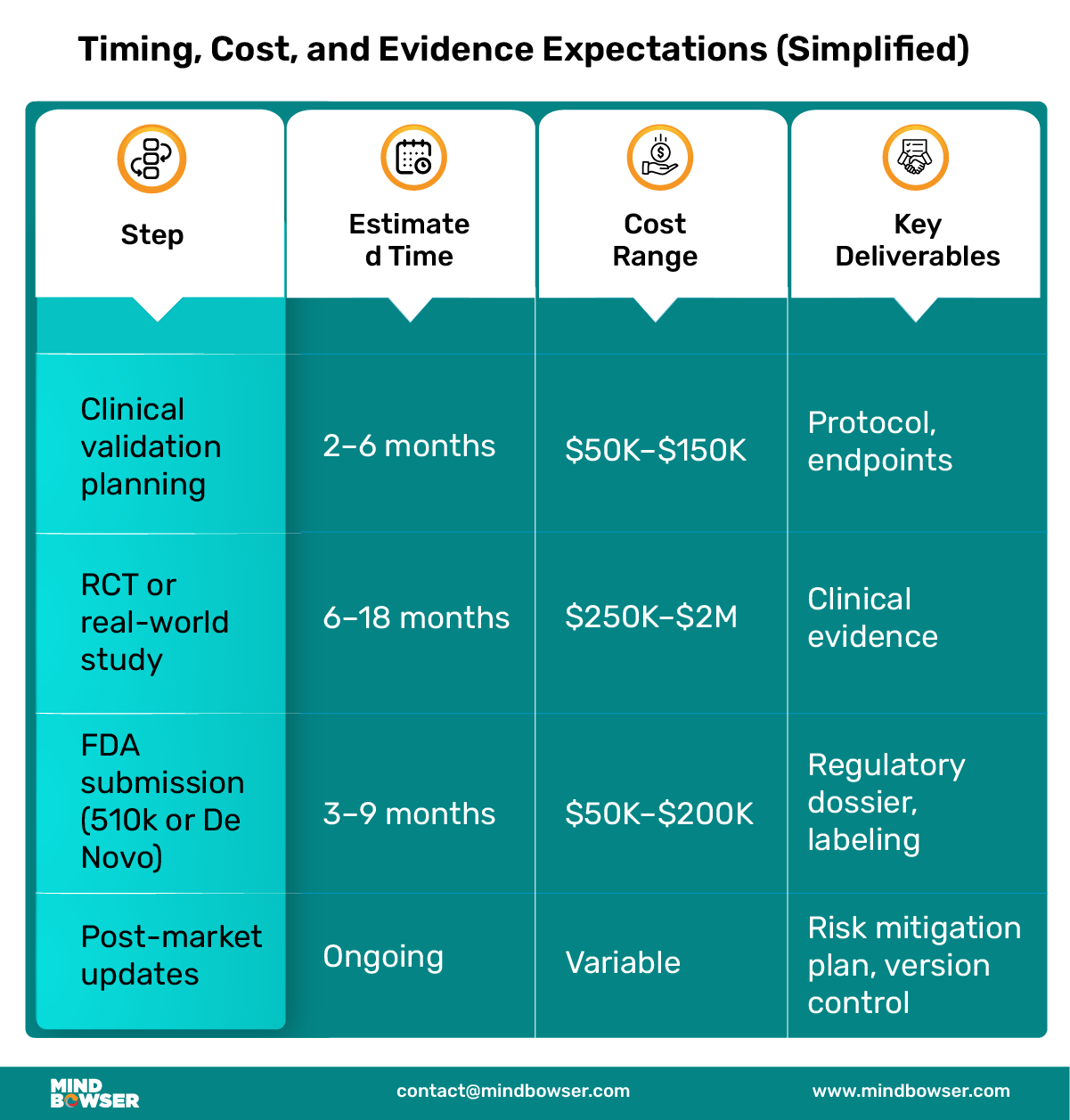
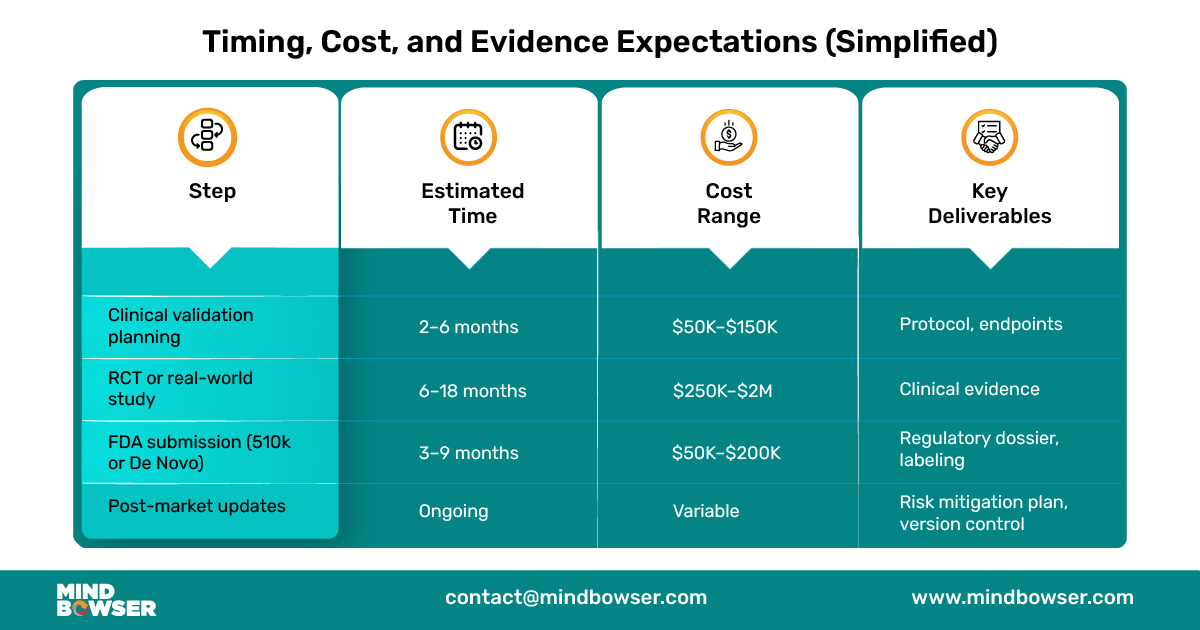
Bringing a prescription digital therapeutic to life requires more than building a great app. It’s a structured, multi-phase journey that blends clinical science, regulatory navigation, and digital product thinking. Below is a product lifecycle breakdown that modern digital health teams can use as a roadmap.
Start by focusing on a specific condition with:
Choose problems where digital interventions can be first-line or adjunct therapy, and where software can deliver measurable outcomes.
Engage clinicians early to validate hypotheses. Define:
Also, data sources, privacy requirements, and patient access needs (e.g., smartphone vs browser, caregiver involvement) should be considered.
Your PDT is both a medical product and a digital experience. Design with:
Before jumping into an RCT, validate your concept through:
This helps refine hypotheses before committing to full-scale validation.
Related read: A Guide To Minimum Viable Architecture Points For Any Startup
Your regulatory strategy will shape your evidence plan. In general:
Make sure your endpoints align with both clinical guidelines and payer expectations.
For a successful submission, you’ll need:
Evidence of usability and software validation
PDTs are still software, and updates are inevitable. Build with:
To bring the theory to life, let’s look at how a real digital health product navigated the path to become a prescription digital therapeutic. One emerging example is MentalBar—a fictionalized but representative case study based on common PDT development patterns. This walkthrough illustrates the blend of clinical, regulatory, and product strategy that goes into building a successful therapeutic.
The team focused on addressing generalized anxiety disorder (GAD) in adolescents—a condition often overlooked due to access gaps and social stigma. They identified the opportunity to develop a validated, self-guided solution that could deliver clinically proven anxiety management tools outside traditional therapy settings.
The product combined:
It offered 10–15 minute daily sessions, optimized for teens, and included clinician-recommended behavioral protocols.
Due to the novelty of the age group and delivery model, the team pursued the De Novo FDA pathway. They worked with regulatory experts to:
A 12-week RCT with over 150 participants demonstrated:
Post-clearance, the company adopted a B2B2C distribution model, including:
This example shows how focused teams can successfully deliver prescription digital therapeutics that are not only compliant but also clinically valuable and commercially viable. With the right roadmap—product, regulatory, and go-to-market strategy can come together to create software that truly heals.
The evolution of digital health has reached a pivotal point. The industry is no longer satisfied with tracking steps or offering general wellness advice. Prescription digital therapeutics are leading the next wave—bridging the gap between software and medicine, and redefining what it means to deliver care through a screen.
For product teams, founders, and innovation leads, the opportunity is clear:
✅ Solve real clinical problems
✅ Earn the trust of providers and payers
✅ Build something that not only engages but truly heals
Whether you’re exploring a new therapeutic category or validating your MVP, starting with the right roadmap reduces risk, speeds up compliance, and creates long-term strategic value. The PDT path is not easy, but for those who can navigate it, the payoff is not just market differentiation—it’s lasting impact.

At Mindbowser, we specialize in helping digital health teams turn clinically validated ideas into market-ready prescription digital therapeutics. Whether you’re just shaping your roadmap or preparing for FDA clearance, we bring the technical, regulatory, and clinical expertise needed to build trusted, scalable products.
🔧 Here’s how we support PDT product development:
Define the right use case, therapeutic model, and product vision with cross-functional input from product, clinical, and regulatory experts.
Build usable, compliant MVPs with patient-centric UX, integrated behavior protocols, and structured data collection.
Collaborate with clinical advisors and CROs to define endpoints, plan feasibility studies, and prepare for RCTs or real-world validation.
Align product development with FDA requirements, including documentation, risk management, and QMS setup.
Leverage our experience in building secure, scalable SaMD platforms integrated with wearable data, EHRs (via HealthConnect CoPilot), and cloud services.
Maintain, iterate, and update your PDT safely with version control, compliance logging, and a dedicated engineering team.

We worked with Mindbowser on a design sprint, and their team did an awesome job. They really helped us shape the look and feel of our web app and gave us a clean, thoughtful design that our build team could...


The team at Mindbowser was highly professional, patient, and collaborative throughout our engagement. They struck the right balance between offering guidance and taking direction, which made the development process smooth. Although our project wasn’t related to healthcare, we clearly benefited...

Founder, Texas Ranch Security

Mindbowser played a crucial role in helping us bring everything together into a unified, cohesive product. Their commitment to industry-standard coding practices made an enormous difference, allowing developers to seamlessly transition in and out of the project without any confusion....

CEO, MarketsAI

I'm thrilled to be partnering with Mindbowser on our journey with TravelRite. The collaboration has been exceptional, and I’m truly grateful for the dedication and expertise the team has brought to the development process. Their commitment to our mission is...

Founder & CEO, TravelRite

The Mindbowser team's professionalism consistently impressed me. Their commitment to quality shone through in every aspect of the project. They truly went the extra mile, ensuring they understood our needs perfectly and were always willing to invest the time to...

CTO, New Day Therapeutics

I collaborated with Mindbowser for several years on a complex SaaS platform project. They took over a partially completed project and successfully transformed it into a fully functional and robust platform. Throughout the entire process, the quality of their work...

President, E.B. Carlson

Mindbowser and team are professional, talented and very responsive. They got us through a challenging situation with our IOT product successfully. They will be our go to dev team going forward.

Founder, Cascada

Amazing team to work with. Very responsive and very skilled in both front and backend engineering. Looking forward to our next project together.

Co-Founder, Emerge

The team is great to work with. Very professional, on task, and efficient.

Founder, PeriopMD

I can not express enough how pleased we are with the whole team. From the first call and meeting, they took our vision and ran with it. Communication was easy and everyone was flexible to our schedule. I’m excited to...

Founder, Seeke

We had very close go live timeline and Mindbowser team got us live a month before.

CEO, BuyNow WorldWide

Mindbowser brought in a team of skilled developers who were easy to work with and deeply committed to the project. If you're looking for reliable, high-quality development support, I’d absolutely recommend them.

Founder, Teach Reach

Mindbowser built both iOS and Android apps for Mindworks, that have stood the test of time. 5 years later they still function quite beautifully. Their team always met their objectives and I'm very happy with the end result. Thank you!

Founder, Mindworks

Mindbowser has delivered a much better quality product than our previous tech vendors. Our product is stable and passed Well Architected Framework Review from AWS.

CEO, PurpleAnt

I am happy to share that we got USD 10k in cloud credits courtesy of our friends at Mindbowser. Thank you Pravin and Ayush, this means a lot to us.

CTO, Shortlist

Mindbowser is one of the reasons that our app is successful. These guys have been a great team.

Founder & CEO, MangoMirror

Kudos for all your hard work and diligence on the Telehealth platform project. You made it possible.

CEO, ThriveHealth

Mindbowser helped us build an awesome iOS app to bring balance to people’s lives.

CEO, SMILINGMIND

They were a very responsive team! Extremely easy to communicate and work with!

Founder & CEO, TotTech

We’ve had very little-to-no hiccups at all—it’s been a really pleasurable experience.

Co-Founder, TEAM8s

Mindbowser was very helpful with explaining the development process and started quickly on the project.

Executive Director of Product Development, Innovation Lab

The greatest benefit we got from Mindbowser is the expertise. Their team has developed apps in all different industries with all types of social proofs.

Co-Founder, Vesica

Mindbowser is professional, efficient and thorough.

Consultant, XPRIZE

Very committed, they create beautiful apps and are very benevolent. They have brilliant Ideas.

Founder, S.T.A.R.S of Wellness

Mindbowser was great; they listened to us a lot and helped us hone in on the actual idea of the app. They had put together fantastic wireframes for us.

Co-Founder, Flat Earth

Mindbowser was incredibly responsive and understood exactly what I needed. They matched me with the perfect team member who not only grasped my vision but executed it flawlessly. The entire experience felt collaborative, efficient, and truly aligned with my goals.

Founder, Child Life On Call

The team from Mindbowser stayed on task, asked the right questions, and completed the required tasks in a timely fashion! Strong work team!

CEO, SDOH2Health LLC

Mindbowser was easy to work with and hit the ground running, immediately feeling like part of our team.

CEO, Stealth Startup

Mindbowser was an excellent partner in developing my fitness app. They were patient, attentive, & understood my business needs. The end product exceeded my expectations. Thrilled to share it globally.

Owner, Phalanx

Mindbowser's expertise in tech, process & mobile development made them our choice for our app. The team was dedicated to the process & delivered high-quality features on time. They also gave valuable industry advice. Highly recommend them for app development...

Co-Founder, Fox&Fork
